※ Browse result(s) for Pupylation
• There are 260 proteins contains lysine Pupylation site(s) in the CPLM database
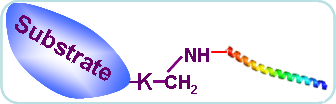
• About lysine Pupylation
Pupylation was recently discovered as the ubiquitous protein post-translational modification on lysine in prokaryotic actinomycetes, which could be reversibly regulated. Comparable with eukaryotes, the prokaryotic ubiquitin-like protein functioned as the tag signaling, while this system as only found in actinomycetes. In contrast to ubiquitination controlled by E1, E2 and E3 in eukaryotes, the pupylation procedure contain two steps. The PUP -GGQ C-terminal is first deamidated to -GGE by Dop/PafD, and then conjugated to specific lysine residues of substrates catalyzed by PUP ligase PafA.